HKUST Develops World’s Most Durable Hydrogen Fuel Cell
A research team led by Prof. SHAO Minhua, Director of HKUST Energy Institute and Professor of Chemical and Biological Engineering have developed a new hydrogen fuel cell which is not only the world’s most durable1 to date, but is also more cost-effective, paving way for a wider application of green energy in the pursuit of a carbon neutral world.
Hydrogen fuel cell is a promising clean energy option as it generates power by converting hydrogen and oxygen into electricity, with zero emission of carbon dioxide, particulate matters and other air pollutants that may cause smog and other health problems. Despite its environmental benefits and years of development, hydrogen fuel cell was still not widely commercialized. That is because its power generation depends heavily on an electrocatalyst - which is largely made up of the expensive and rare metal platinum.
Scientists have strived to develop alternatives by replacing platinum with more common and inexpensive materials like iron-nitrogen-carbon, but those materials are either proven inefficient in power generation or have poor durability.
Now, Prof. Shao's research team found a new formula which not only could cut down the proportion of platinum used by 80 percent, but also set a record in terms of the cell’s durability level.
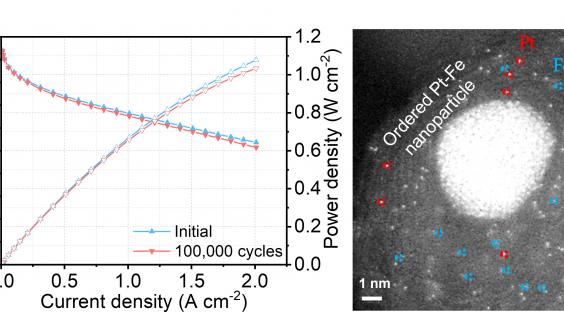
Despite a low portion of platinum, the new hybrid catalyst developed by the team managed to maintain the platinum catalytic activity at 97% after 100,000 cycles2 of accelerated stress test, compared to the current catalyst which normally sees a drop of over 50% in performance after 30,000 cycles. In another test, the new fuel cell did not show any performance decay after operating for 200 hours3.
One reason behind such outstanding performance was the fact that the new catalyst has three different active sites for the reaction, instead of just one in current catalysts. Using a formula containing atomically dispersed platinum, iron single atoms and platinum-iron nanoparticles, the new mix accelerates reaction rate and achieves a catalytic activity 3.7 times higher than the platinum itself. Theoretically, the higher the catalytic activity, the greater the power it delivers.
Prof. Shao, also the Director of HKUST Energy Institute, said, “Hydrogen fuel cell is an energy conversion device essential for our aspiration of achieving a carbon neutral world, there is a need to expand its use amidst our fight against climate change. We are delighted to see our research findings bringing this goal a step closer. Thanks to the Government’s Green Tech Fund, we will seek to further refine the catalyst and make it compatible with fuel cell vehicles and other electrochemical devices.”
The study was financially supported by National Key R&D Program of China, Shenzhen Science and Technology Innovation Committee, and the Research Grant Council of the Hong Kong Special Administrative Region. The research findings were recently published in the journal Nature Catalysis.
1According to the test protocols of US Department of Energy in assessing the durability of fuel cell
2One cycle is equivalent to 3 seconds with voltage level at 0.6V, followed by another 3 seconds with voltage level at 0.9V
3The voltage level is set at 0.6V